
Low-temperature methanolysis of polycarbonate over solid base sodium aluminate
Philip Anggo Krisbiantoro (Prof. Kevin C.-W. Wu’s group)
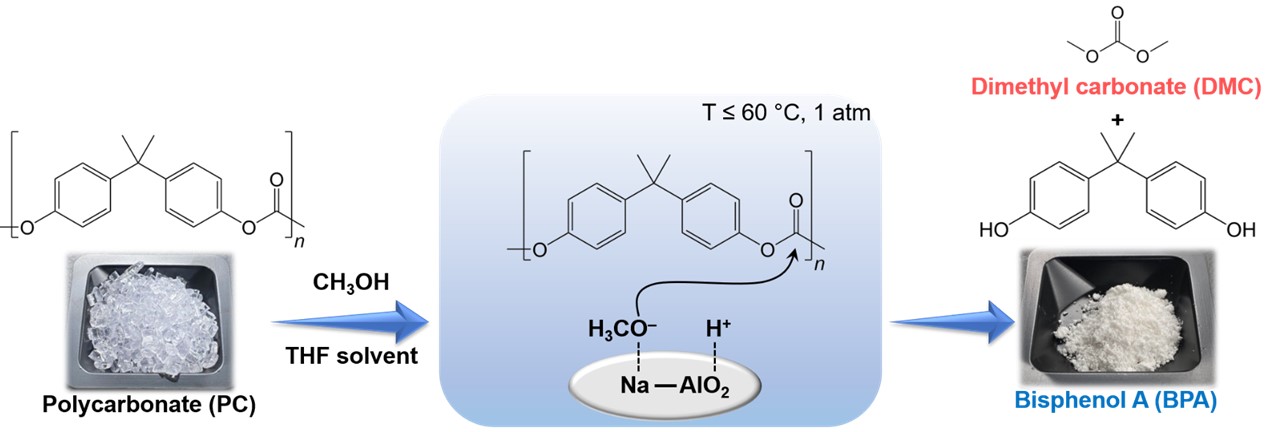
Herein, a low-cost and readily available sodium aluminate (NaAlO2) was used as a solid base catalyst for the depolymerization of polycarbonate (PC) via methanolysis in the presence of tetrahydrofuran (THF) as a solvent. NaAlO2 was highly active for the reaction, and the performance was comparable to that of soluble strong base SrO and much higher than those of MgO and CaO. By the reaction over the catalyst, a highly pure and crystalline bisphenol A (BPA) was obtained. Among tested organic solvents, THF was the best in aiding PC methanolysis over NaAlO2 due to the polarity similar to PC according to Hansen solubility parameters (HSPs). At 60 °C, 98.1% PC conversion and 96.8% BPA yield were achieved within just 2 h. NaAlO2 was reusable without any severe catalyst deactivation in at least four runs. The mechanistic study revealed that the reaction proceeded via the methoxide pathway, with THF aiding the dissolution of PC. The reaction over NaAlO2 possessed a low apparent activation energy (Ea) of 75.1 kJ mol–1, which is the lowest ever reported so far for the reaction over solid catalysts.
This work has been published in Langmuir, 2024, 40, 10, 5338–5347.



