
PET-derived bis(2-hydroxyethyl) terephthalate as a new linker source for solvent-free and hydrothermal synthesis of BDC-based MOFs
Philip Anggo Krisbiantoro (Prof. Kevin C.-W. Wu’s group)
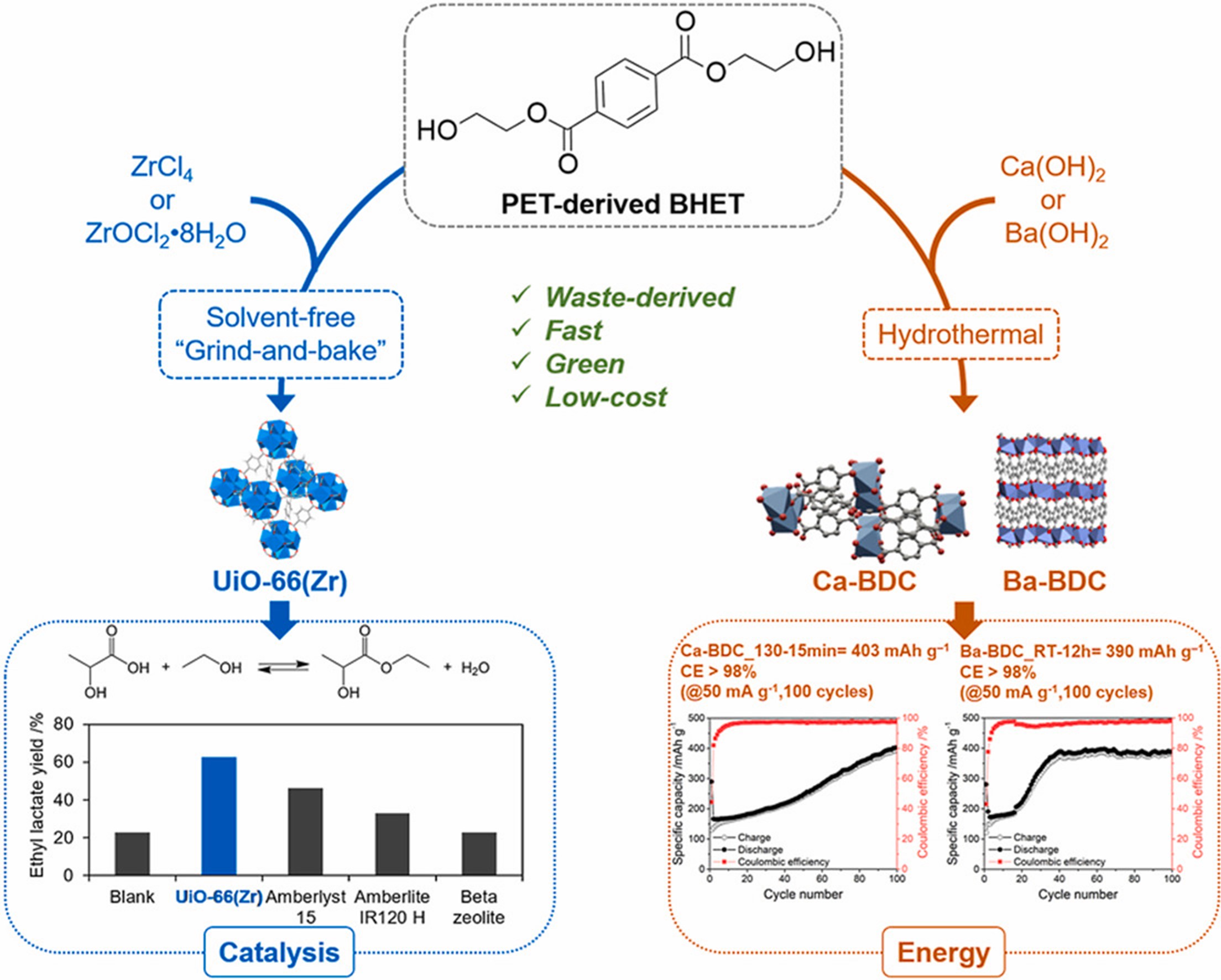
To date, the high cost of organic linkers and the energy-consuming synthesis processes remain two of the main challenges for the commercialization of metal-organic frameworks (MOFs). Herein, we demonstrate that polyethylene terephthalate (PET)-derived bis(2-hydroxyethyl) terephthalate (BHET) is a new linker source that enables the facile solvent-free and hydrothermal synthesis of BDC-based MOFs. Using BHET as a linker source, UiO-66(Zr) was rapidly synthesized via a solvent-free “grind and bake” technique, while Ca-BDC and Ba-BDC were easily obtained by using hydrothermal synthesis. We found that the hydrolysis of BHET to terephthalate anion (BDC2−) over proton produced from the hydrolysis/clustering of Zr precursor and hydroxyl anion produced from the dissolution of M(OH)2 (M= Ca or Ba) was the key to the crystal growth of solvent-free synthesized UiO-66(Zr) and hydrothermally synthesized M-BDC (M= Ca or Ba), respectively. While the as-synthesized UiO-66(Zr) was highly active for the esterification of lactic acid (LA) with ethanol (EtOH), Ca-BDC and Ba-BDC exhibited remarkable electrochemical performance for lithium storage. Our strategy provides a major step towards realizing the idea of a more facile, green, and low-cost synthesis of PET-derived MOFs compared to prior arts applicable for catalysis and energy applications.
This work has been published in Materials Today Nano, 2024, 25, 100459.
https://www.sciencedirect.com/science/article/pii/S2588842024000099#sec5



